Abstract
Background
Adoptive cell therapy of multiple myeloma is limited in part by T cell metabolic fitness, which can be enhanced by mTOR inhibition during ex vivo manufacturing. In a first-generation trial using ex vivo rapamycin, polyclonal autologous Th1/Tc1 (RAPA-101) cells were safe and associated with delayed relapse when administered after hematopoietic cell transplantation in high-risk MM patients. Now, we are evaluating temsirolimus for manufacture of second-generation RAPA-201 cells. Relative to RAPA-101, RAPA-201 have enhanced Th1/Tc1 polarity, reduced immune checkpoints, and improved response to the key homeostatic cytokines IL-7 and IL-15 [Cytotherapy 23 (2021) S86, abstract 403]. Here, we report the initial results of RAPA-201 cell therapy, which is being evaluated on a phase 2 clinical trial in adult patients (pts) with triple class refractory MM (NCT04176380).
Methods
Trial accrual was limited to MM pts who received ≥ 3 prior lines of therapy, including an anti-CD38 monoclonal antibody. All pts were refractory to a proteasome inhibitor, an immunomodulatory agent, and the last treatment line. RAPA-201 were manufactured from an autologous pheresis product using a one-week culture in media containing temsirolimus (3 µM) and IFN-a (20,000 IU/mL). Bridging chemotherapy during manufacturing (Cycle 1) and host conditioning prior to RAPA-201 infusion consisted of the 14-day PC regimen [pentostatin (4 mg/m 2 IV; days 1, 4, 8, 12; dose adjusted/omitted with renal insufficiency); cyclophosphamide (100-200 mg PO, days 1-5 and days 8-12)]. Cryopreserved RAPA-201 were thawed and administered every 35-days for up to four additional cycles (Cycles 2-5). The primary study objective was overall response rate (ORR). Efficacy of polyclonal T cell therapy may require extensive in vivo clonal expansion; to assess this, TCR sequencing was performed pre- and post-therapy.
Results
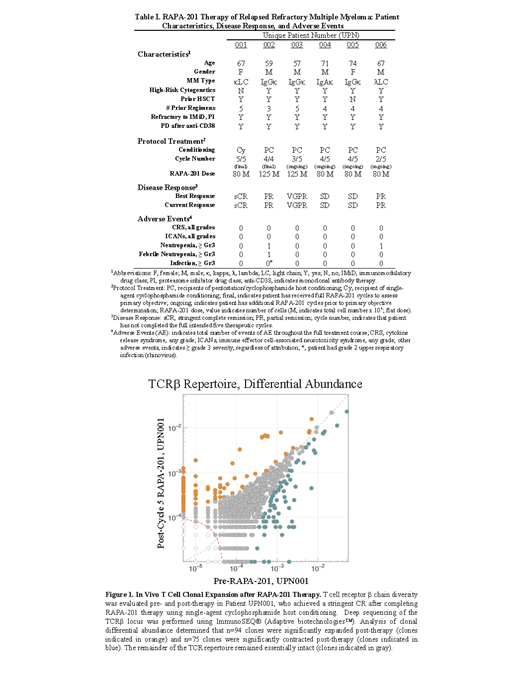
As of 01 August 2021, 6 pts have enrolled. RAPA-201, which were successfully manufactured in each pt, contained a mix of CD4 + and CD8 + T cell subsets that were quiescent (negligible CD25), enriched for T central-memory characteristics (CD62L/CCR7 co-expression; responsive to IL-7/IL-15), had negligible checkpoint expression (including PD-1), and had preferential type I cytokine secretion (IFN-g, GM-CSF, TNF-a) with negligible type II cytokine secretion (IL-4, IL-5, IL-10, and IL-13). Table 1 describes pt demographics, adverse events (AE), and disease responses. Pts were heavily pretreated with a median of 4 prior lines (range, 3-5). Twenty-two treatment cycles were administered exclusively on an outpatient basis. Treatment cycles were safely administered, as evidenced by the following AE incidences: CRS (0/22); ICANS (0/22); neutropenic fever (1/22); and serious infection (0/22). Two pts have completed therapy, with each meeting the primary disease response objective. Four pts are receiving ongoing therapy, two of whom have already met the primary disease response objective. Disease response to RAPA-201 therapy was associated with extensive in vivo T cell clonal expansion, as Pt. UPN001, who achieved stringent complete response (sCR), had n=94 T cell clones expanded post-therapy (Figure 1).
Conclusions
Autologous RAPA-201 were successfully manufactured, administered with extraordinary safety, and yielded clinical responses in RRMM, including a stringent complete remission. RAPA-201 therapy represents a new paradigm that utilizes stringent mTOR inhibition to reprogram Th1/Tc1 cells for enhanced metabolic fitness and induction of in vivo T cell clonal expansion, thus providing an alternative to gene-modified targeted T cell therapy. With these promising safety and efficacy results, current RAPA-201 developmental efforts are directed towards completing protocol accrual in parallel with the design and implementation of next-generation clinical trials.
Dhakal: BMS: Honoraria, Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Karyopharm: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Natera: Membership on an entity's Board of Directors or advisory committees; Carsgen: Research Funding; Fate: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lum: Transtarget, Inc.: Other: Co-founder. D'Souza: Sanofi, Takeda, Teneobio, CAELUM, Prothena: Research Funding; Janssen, Prothena: Consultancy; Imbrium, Pfizer, BMS: Membership on an entity's Board of Directors or advisory committees. Chhabra: GSK: Honoraria. Fowler: Rapa Therapeutics: Current equity holder in publicly-traded company, Patents & Royalties. Hari: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal